Research Progress
Greenhouse gas carbon dioxide (CO2) is widely considered to be responsible for the climate change, and its utilization as an alternative carbon feedstock may be a viable approach for its remedy. Consequently, electrochemical conversion of CO2into value-added chemicals or fuels has attracted significant interest, although it suffers from a large overpotential and low selectivity.
Recently, a joint research team from CAS Key Laboratory of Low-Carbon Conversion Science & Engineering of Shanghai Advanced Research Institute (SARI) and SARI-ShanghaiTech University Joint Lab has found a new electrocatalyst to exclusively convert CO2 to formic acid, a chemical widely used in medical, chemical and agricultural lines all over the world over the supported Pd-Sn alloy, and thereby realizing the utilization of CO2 as an affordable carbon resource. This is attributed to the tuning of surface electronic structures of supported Pd-Sn alloy NPs. The electrocataytic activity and selectivity are highly dependent on the surface configurations, in which formic acid with the faradaic efficiency of 99% at the lowest overpotential of -0.26 V was produced on the PdSn alloy surface with optimal surface Pd, Sn and O configuration.
On the other hand, the research team has developed a class of mesoporous nitrogen-doped carbon (N-Carbon) tailored with highly uniform cylindrical channel structures to dramatically boost C-C bond formation in CO2 electroreduction. The as-prepared metal-free N-carbon catalyst can convert CO2 into ethanol with the faradaic efficiency as high as 77% at the low potential of ?0.56 V (vs. RHE). As the competitive CO2 reduction into carbon monoxide or other products was completely suppressed, an almost 100% selectivity to ethanol was achieved. This work will open up an avenue for developing robust metal-free carbon-based electrocatalysts for converting CO2 into C2 compounds with high selectivity and efficiency.
The latest result was published in the famous scientific journal Angewandte Chemie International Edition (doi: 10.1002/anie.201707098; doi: 10.1002/ange.201706777).
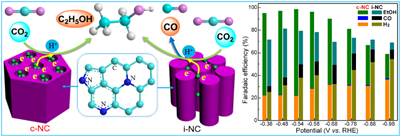
Electrocatalytic conversion of CO2 into ethanol over nitrogen-doped carbon catalysts (Image by SARI)
This work was supported by the Hundred Talents Program of Chinese Academy of Sciences, the Ministry of Science and Technology, the SARI-ShanghaiTech Low-carbon Joint Lab, and the SARI Innovation Fund.





