Research Progress
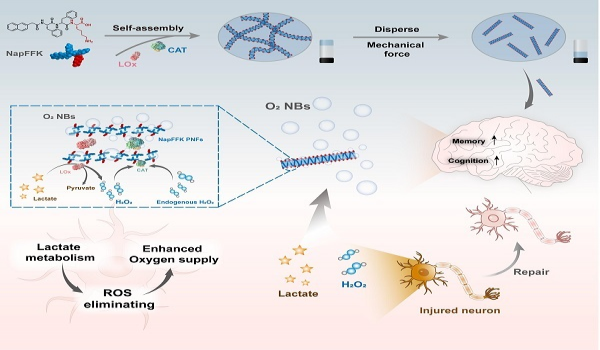
Researchers Propose a Dynamic Formation Mechanism of Enzymatic Nanobubbles for Nerve Injury Treatment
A joint research team led by Prof. ZHANG Lijuan from the Shanghai Advanced Research Institute, Chinese Academy of Sciences and Tongji University proposed a peptide-stabilized lactate oxidase and catalase system, which can effectively deliver oxygen to cells and tissues in Nerve Injury Treatment. The findings were published in Cell Biomaterials.
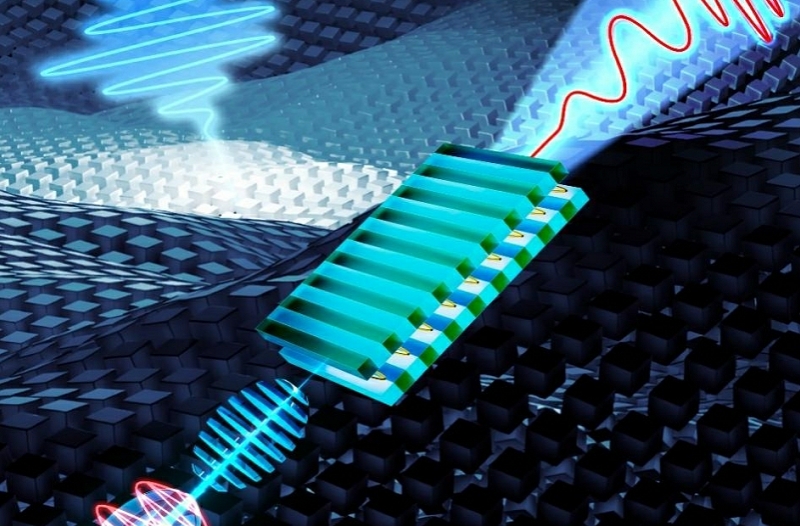
Researchers Achieve 7-30 THz Continuously Tunable Strong-Field Terahertz Free-Electron Lasers
In a study published in Nature Photonics, the Free-electron Laser Team of the Shanghai Advanced Research Institute (SARI), Chinese Academy of Sciences developed an innovative approach by employing beating-frequency laser modulation of electron beams to precisely tailor the beam structure and produce continuously tunable THz radiation.
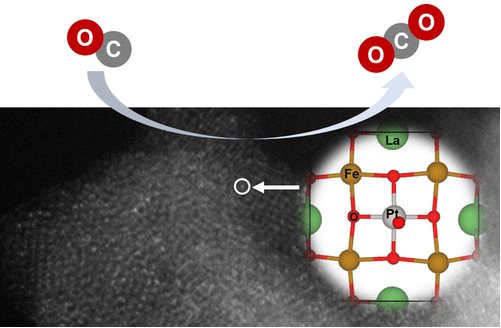
Researchers Developed High-performance Pt Single-Atom Catalysts for Low-Temperature CO Oxidation
A research team from Shanghai Synchrotron Radiation Facility (SSRF) Science Center of Shanghai Advanced Research Institute, Chinese Academy of Sciences, have developed a high-performance catalyst by anchoring oxidized platinum single atoms on La-vacancy LaFeO₃ via a defect-engineering strategy. The catalyst delivers efficient low-temperature CO oxidation without reduction pretreatment and shows remarkable stability. The findings were published in the Journal of the American Chemical Society.
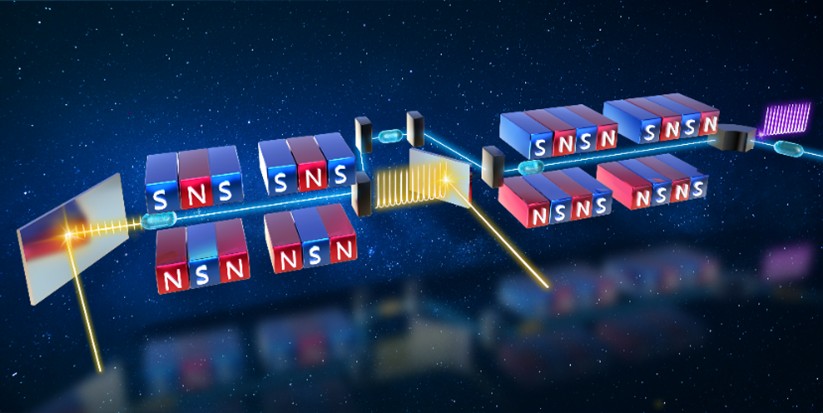
Researchers Proposed a Direct-Amplification Enabled Harmonic Generation Free-Electron Laser
Recently, the Free-Electron Laser (FEL) team at the Shanghai Advanced Research Institute (SARI), Chinese Academy of Sciences experimentally demonstrated their independently proposed concept of Direct-Amplification Enabled Harmonic Generation Free-Electron Laser (DEHG-FEL) for the first time, and successfully achieved its lasing and stable operation.
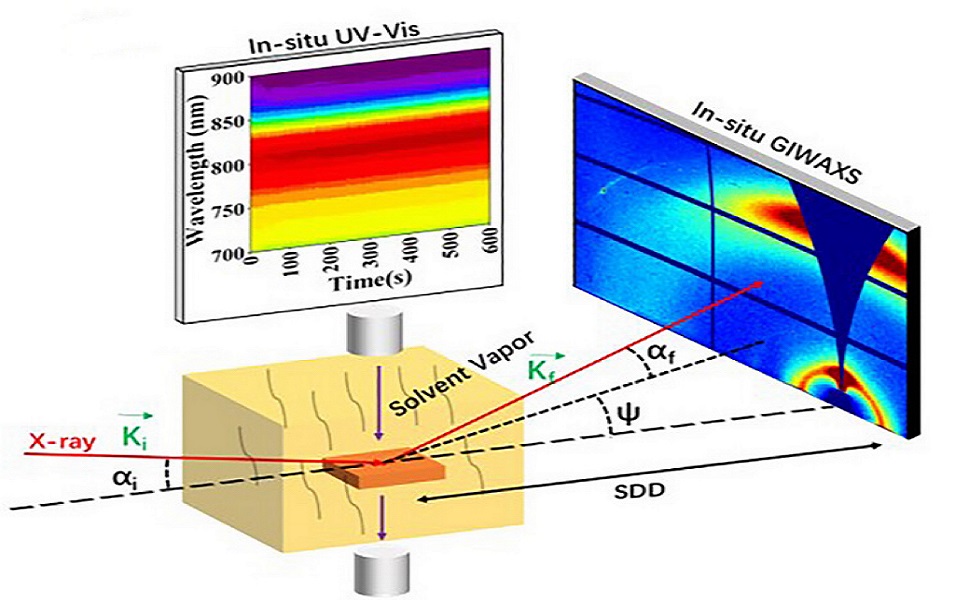
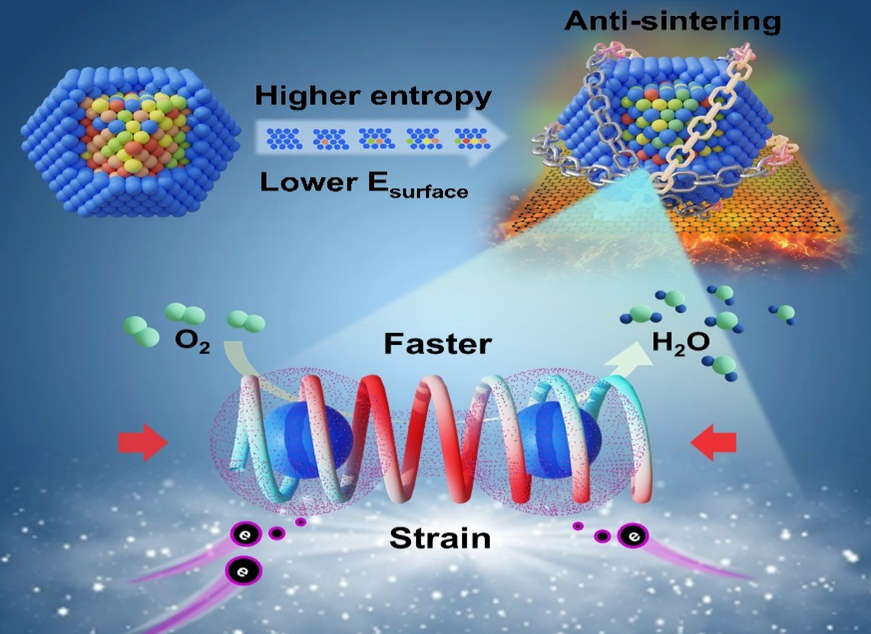
Researchers Found Novel Approach to Low-loading Pt Based Catalyst for PEM Fuel Cell
A research team at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences proposed an entropy-increase assisted anti-sintering concept to fundamentally reduce the surface energy of NPs by increasing the mixing entropy, thus hindering the migration and coalescence of NPs. The research results were published in Advanced Functional Materials in May, 2025.





