Research Progress
An international joint research team from Zhejiang University, Shanghai Advanced Research Institute of the Chinese Academy of Sciences, and Technical University of Denmark, reported an in-situ strategy to manipulate interfacial structure with atomic precision during catalytic reactions. Results were published in the latest issue of Science.
The interface between nanoparticles and substrates plays a critical role in heterogeneous catalysis because most active sites are located at the perimeter of the interface. It is generally believed that this interface is immobile and unchangeable, thus can hardly be adjusted in reactive environments. As a result, it has been challenging to promote catalytic activity through precise control of the interfacial structure.
In this study, the scientists first used environmental transmission electron microscopy to directly visualize the epitaxial rotation of gold nanoparticles on titanium dioxide (TiO2) surfaces during CO oxidation at the atomic level. A perfect epitaxial relationship was observed between Au nanoparticles and TiO2 (001) surfaces under an O2 environment in real time.
Theoretical calculations including density functional theory calculations and thermodynamics analysis were then carried out, indicating that the epitaxial orientation could be induced by changing O2 adsorption coverage at the perimeter interface. The Au nanoparticle was more stable with adsorption of more O2 molecules at the Au-TiO2 interface, but became less stable with the consumption of O2 with CO.
To exploit the promoted activity of Au-TiO2 interface, researchers conducted additional top-view observations and found that this configuration remained unchanged when cooling from 500 °C to 20 °C in CO and O2 reactive environments, showing the rotation of the Au nanoparticle was also temperature dependent in reaction conditions.
Taking advantage of the reversible and controllable rotation of the Au nanoparticle, the scientists achieved in-situ manipulation of the active Au-TiO2 interface at the atomic level by changing gas and temperature.
This study sheds light on real-time manipulation of catalytic interface structure in reaction conditions at the atomic scale, which may inspire future approaches to real-time design of the catalytic interface under operating conditions.
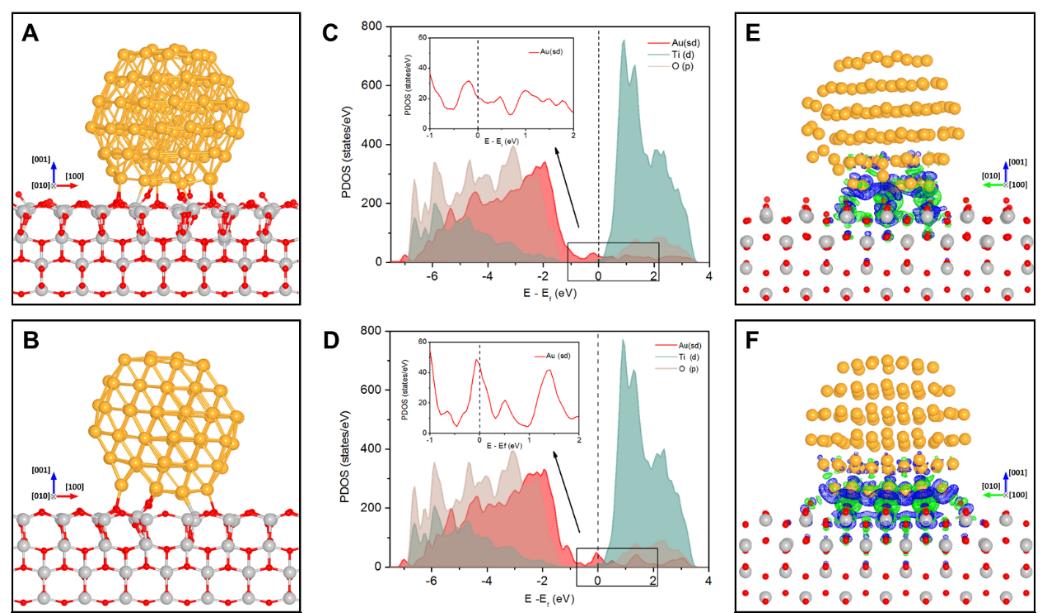
Figure 1. Geometric and electronic structure of Au-TiO2 interface under CO/O2 (A, C, E) and O2 environment (B, D, F) (Image adapted from Science)
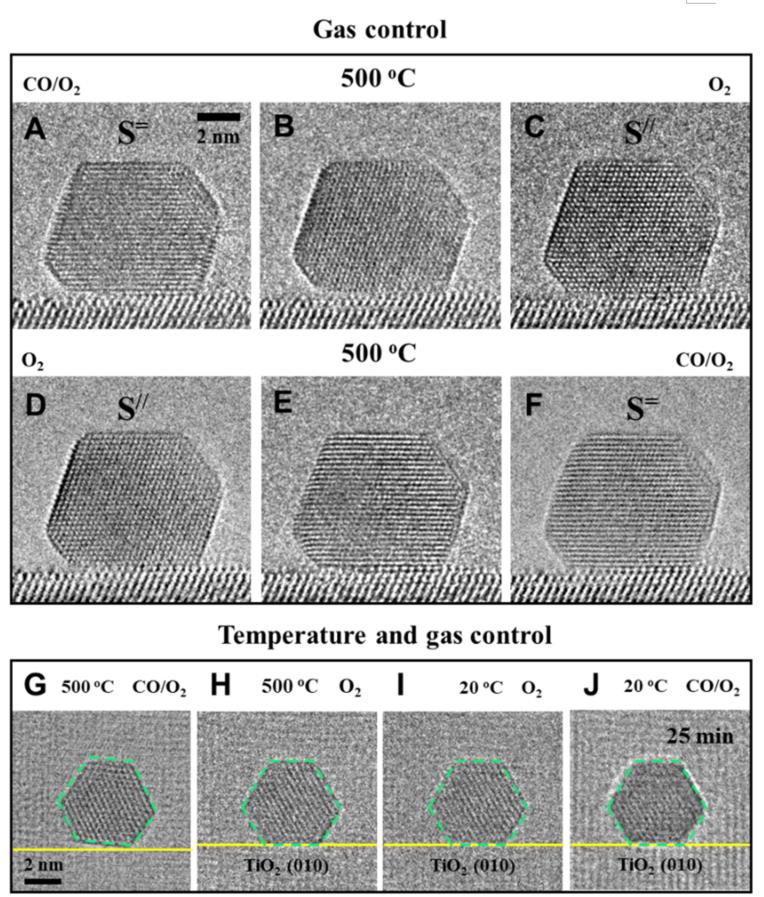
Figure 2. Manipulation of the Au-TiO2 interface using temperature and gas control (Image adapted from Science)
Figure 3. Schematic illustration of In-situ manipulation of active Au-TiO2 interface (Credited by Yong Wang, Zhejiang University)
Contact: GAO Yi
Shanghai Advanced Research Institute, Chinese Academy of Sciences
Email: gaoyi@zjlab.org.cn





