Research Progress
Direct conversion of methane to oxygenates has attracted numerous research interests from both industrial and academics. However, selective oxidation of methane to value-added chemicals with both high catalytic activity and selectivity under mild conditions still remains a great challenge.
Firstly, selective oxidation of CH4 to oxygenates with O2 or O2/H2 suffers from low catalytic activity and low oxygenates selectivity, due to the low activity of oxygen and the overoxidation of the oxygenates. Secondly, the high loading (1~5 wt%) of noble metals for supported catalysts used for methane conversion leads to high cost, which is not business-friendly.
Inspired by these challenges, a research team led by Prof. SUN Yuhan and Prof. ZHONG Liangshu at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a ZSM-5 (Z-5) supported PdCu catalyst for selective oxidation of CH4 to oxygenates using O2 in the presence of H2. The as-obtained PdCu/Z-5 catalyst exhibited a high oxygenates yield of 1178 mmol/gPd/h with oxygenates selectivity of 95% at 120 °C. The research results were published in the latest issue of Angew. Chem. Int. Ed.
Based on a combination of control experiments and electron paramagnetic resonance as well as in situ spectroscopic techniques, researchers found that PdO nanoparticles facilitated in situ generation of H2O2, while Cu single atoms not only accelerated the generation of abundant ·OH from H2O2 decomposition, but also enabled the homolytic cleavage of CH4 by ·OH to ·CH3. Subsequently, the ·OH reacted quickly with the ·CH3 to form CH3OH with high selectivity.
These findings may provide valuable insights into selective oxidation of methane to oxygenates, which may also shed light on other highly efficient and low-price catalysts for the selective activation of C-H bonds in light alkanes.
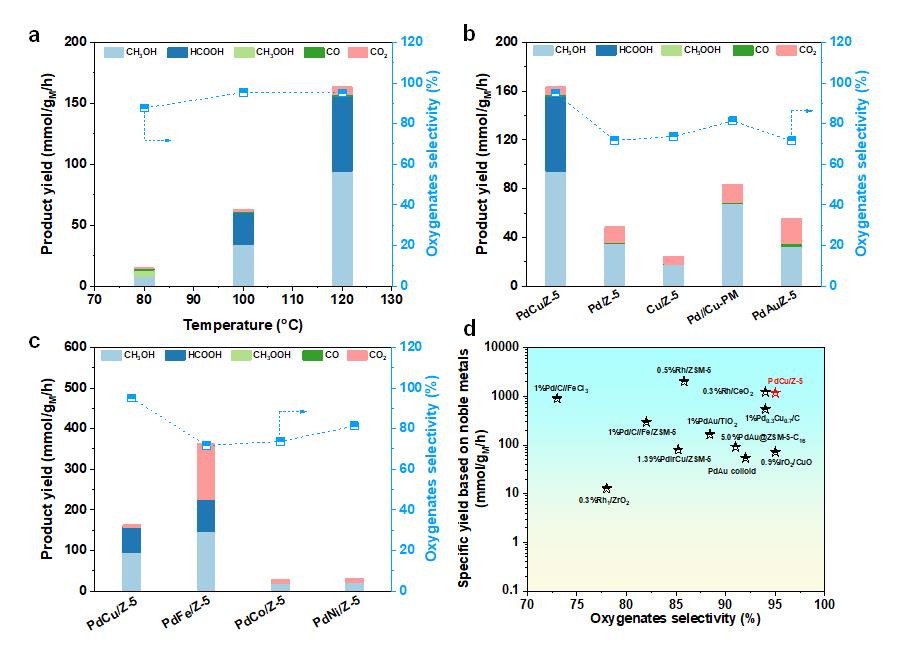
Product yield and oxygenates selectivity for selective oxidation of CH4 with O2 and H2. (Image by SARI)





