Research Progress
However, due to the absence of quantitative analysis of the reaction dynamics and an understanding of gas transport mechanism at the solid–liquid–gas interface, a comprehensive understanding of gas transport in liquid and following reactions at the triple-phase interfaces remains unclear.
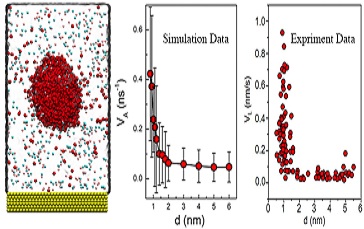
Motivated by this challenge, Prof. CHEN Jige at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences, collaborated with Prof. FANG Haiping at East China University of Science and Technology, Prof. Sun Litao at Southeast University, and Prof. ZHENG Haimei at Lawrence Berkeley National Laboratory, reported a real-time observation of the accelerated solid-liquid-gas etching progress of gold nanorods by introducing gas nanobubbles and revealed the underlying microscopic mechanism dependent on liquid layer thickness. The results were published in the latest Nature Materials.
In this work, the real-time observation of the accelerated etching of gold nanorods with oxygen nanobubbles in aqueous hydrobromic acid is given by using liquid-cell transmission electron microscopy (TEM). It is found that when an oxygen nanobubble is close to a nanorod below the critical distance (~1 nm), the local etching rate is significantly enhanced by over one order of magnitude. Molecular dynamics simulation results reveal that the strong attractive van der Waals interaction between the gold nanorod and oxygen molecules governs oxygen transport through the thin liquid layer and thus leads to enhanced etching rate.
The finding of a critical distance for etching acceleration between nanobubbles and gold nanorods leads to dramatical different physics picture, which is different from the conventional view that a more rapid reaction of nanobubbles toward the solid relates to a more rapid reaction.