Research Progress
As one of the largest-volume industrial chemical processes today, hydroformylation converts olefins, H2 and CO into aldehydes and related products more than 10 million tons annually.
Although Au exhibits good ability towards olefins activation, H2 dissociation and CO bonding, it is conventionally considered inactive for hydroformylation due to its intrinsic inertness.
Now, a research team led by Profs. WANG Hui and SUN Yuhan from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences designed a zeolite-encaged Au single-atom catalyst with Au1-O-SiOX motifs, which shows remarkable catalytic activity and selectivity towards propene hydroformylation.
The study was published in Chem Catalysis on July 13.
Preliminary performance evaluation of impregnated Au on zeolite demonstrates that sub-nanometer Au clusters exhibit higher activity than nanoparticles in hydroformylation. Inspired by this, the confinement effect of zeolite is utilized to regulate the particle size of Au. Nanoparticles/sub-nanoclusters and atomically dispersed Au species within zeolite can be unambiguously observed through high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM).
The Au1@S-1 catalyst shows a total 3,794 μmol butyraldehyde and noticeable stability after 5 cycles, which is about one order of magnitude more active than Au nanoparticles and is even comparable to Rh-based catalysts.
Detailed characterizations and theoretical calculations indicate that the isolated Au atoms within the zeolite matrix are stabilized via oxygen bridge bonds. The formed Au1-O-SiOX motifs render maximum active site density and high structural stability, which are identified as the real active sites for efficient hydroformylation.
This work makes conventionally inactive Au efficient alternative for hydroformylation by reasonably tailoring the size, contact structure and electronic environment of active metals on specific reactions.
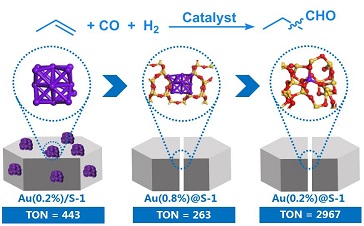
Structural modeling and performance comparison of Au-based catalysts (Image by SARI)





