Research Progress
The electrochemical conversion of CO2 into carbon-based fuels and valuable feedstocks by renewable electricity is an attractive strategy for carbon neutrality. CO is the key component of syngas, a mixture of CO and H2 that can be directly converted into various value-added chemicals via well-developed industrial processes such as Fischer-Tropsch synthesis, methanol synthesis, etc. Therefore, CO2 electroreduction to CO is considered to be one of the most promising routes to obtain cost-competitive products.
However, due to the low solubility and diffusion coefficient of CO2 in aqueous electrolytes, it remains a challenge to possess a large current density, a high faradaic efficiency and excellent stability for practical applications of CO2 utilization.
Motivated by such a challenge, a research team led by Profs. CHEN Wei and WEI Wei from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a facile tactic that enables exceedingly efficient CO2 electroreduction to CO by virtue of low-coordination chloride ion (Cl-) adsorption on a silver hollow fiber (Ag HF) electrode.
The results were published in Angew. Chem. Int. Ed. on September 3.
On the basis of the high efficient electroreduction CO2 to CO over silver hollow fiber (Ag HF) electrode, the research team further introduced chloride ions into the electrode solution, by means of specific adsorption of chloride ions, the electronic structure of the electrode surface was functionally regulated to inhibit the side reaction of hydrogen evolution (HER).
The low-coordination chloride ion (Cl-) adsorption on a silver hollow fiber (Ag HF) electrode reduces CO2 to CO at a stable (>150 h) ampere-level current densities (1 A·cm-2) with a high CO faradaic efficiencies (>92%).
Electrochemical experiments, operando Raman combined with density functional theory (DFT) calculations demonstrated that the high concentration Cl- in the electrolyte could be low-coordination adsorbed onto the surface of Ag HF, which not only hinders the occurrence of the HER, but also optimizes the kinetics of CO2 reduction to CO, leading to a better eCO2RR performance, even at the ampere-level current density.
This work provides a new strategy for further developing electrocatalytic CO2 systems with high current density, high selectivity and high stability in CO2utilization and chlor-alkali industry.
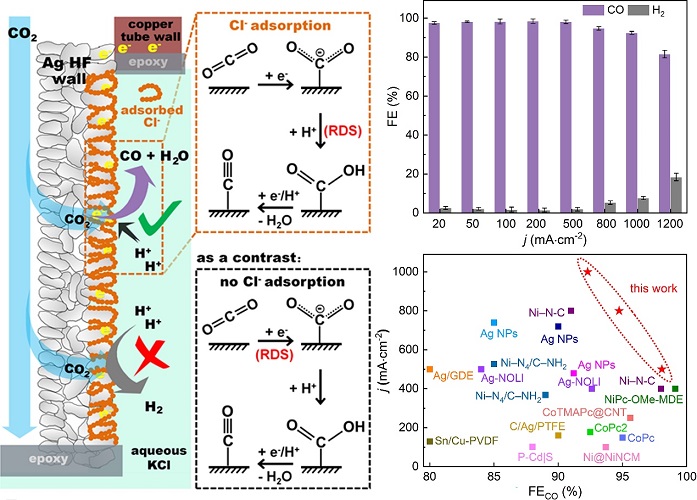
Schematic diagram and electrocatalytic performance of efficient CO2 reduction over silver hollow fiber electrode with chloride ion adsorption (Image by SARI)





