Research Progress
Researchers Constructed Noninterpenetrated Three-dimensional Covalent Organic Framework for Au Ions Capture
date:
2023-04-28
Covalent organic frameworks (COFs) can be ideal platform for detecting or extracting metal ions because of the different functional building units and large surface area. However, most of 3D COFs have interpenetration because of the existence of non-covalent interactions between the adjacent nets, which resulting in decreased surface areas and porosizes, and thus limited their applications in catalysis and molecular/gas adsorption.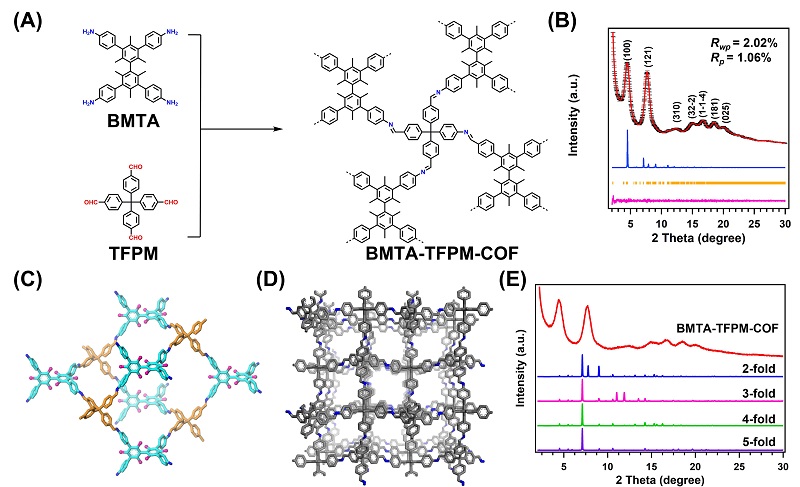
Recently, a research team led by Prof. ZENG Gaofeng and Associate Prof. XU Qing at the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences constructed a novel non-interpenetration 3D COF towards Au ions capture by imine bonds in the frameworks. The high surface area and abundant cavities due to the non-interpenetration provided the high Au3+ capacity (570.18 mg g-1), selectivity (99.5%) and efficiency (68.3% adsorption of maximum capacity in 5 mins).
The synthesized BMTA-TFPM-COF displayed good crystallinity with dia topology and a high BET surface area of 1924 m2 g-1. Importantly, the open cavities and exposed C=N bonds from non-fold interpenetration contributed to high capacity, selectivity and stability of Au3+ uptake.
The experiments showed the mechanism of Au capture. The protonated C=N bonds due to the influence of the HAuCl4 and the protonated nitrogenous groups could adsorb AuCl4 - and reduce Au(III) to Au(I) and Au(0) in acidic solution. Thus, the BMTA-TFPM-COF with abundant exposed C=N bonds could promote the conversion from Au(III) to Au(I) and Au(0) through the protonated C=N bonds, which further verified that the C=N bonds could participate in the reduction of Au(III).

Mechanism of 3D COFs (Image by SARI)
This study gives new insight into the development of 3D COFs for Au3+ capture.





