Research Progress
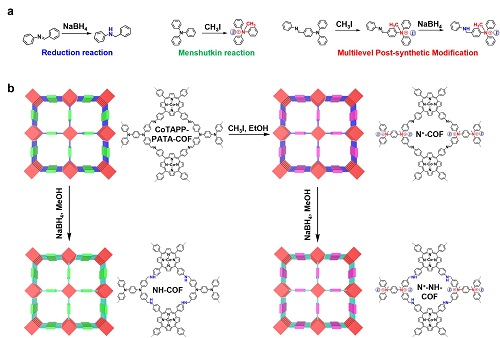
The C-N bonds can improve the adsorption of CO2 and ionic skeletons can promote the charge transfer, further enhancing the conductivity. However, directly bottom-up synthesis can hardly realize the co-existence of C-N bonds and ionic frameworks due to the electrostatic repulsion and weak strength of the linkage.
Motivated by this challenge, a research team led by Prof. ZENG Gaofeng and Associate Prof. XU Qing at the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences constructed a multilevel post-synthetic modification strategy to construct catalytic COFs towards CO2RR with high activity and selectivity. The research results were published in Nature Communications.
The catalytic COFs synthesised by the post modification show a maximum turnover frequencie value of 9922.68 h–1 at –1.0 V and the highest faradaic efficiencies of 97.32% at –0.8 V, which are higher than that of the base COF and the single-modified COFs. The electrocatalysis tests and characterizations reveal that C-N bonds can improve the catalytic selectivity and ionic skeleton contributes to higher activity.
Furthermore, the theoretical calculations illustrate that the easier formation of immediate *CO from COOH* is the rate determined step, and methyl groups strengthen the electron density.
This work provides a deeper understanding of COFs in CO2 reduction reaction. In the meantime, it sheds light on constructing multilevel post-synthetic modification COFs towards tailored activity and high stability.
Catalytic covalent organic frameworks with bifunctional roles in oxygen reduction reaction (from O2 to H2O) and oxygen evolution reaction (from H2O to O2) have been first demonstrated by integrating redox-active sites into the Co-porphyrin frameworks. (Image by SARI)





