Research Progress
A research team led by Prof. GAO Peng and Prof. LI Shenggang at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences developed an efficient FeZn-based catalyst for methanol synthesis via CO2 hydrogenation. After investigating the crystal structure engineering of FeZn catalysts, the research team reported for the first time that ZnFe2O4 spinel enables high methanol selectivity of 84.5% in CO2 hydrogenation.
The research results were published in the latest issue of Chem.
The catalytic conversion of CO2 into alcohols using low-cost green hydrogen is a promising solution for mitigating CO2 emissions. Substantial progress has been made in developing efficient CO2-to-methanol catalysts and revealing the reaction mechanisms, in which highly selective CO2 hydrogenation into alcohols remains a great challenge, due to the difficulty in controlling the C-C coupling steps.
Noble metal-based catalysts were reported to exhibit high ethanol selectivity in CO2 hydrogenation to higher alcohols containing two or more carbon atoms (C2+OH). The research team investigated how crystal structure engineering of FeZn catalysts transformed selective product formation from methanol to C2+OH, realizing unprecedented higher alcohol synthesis (HAS) performance from CO2 hydrogenation.
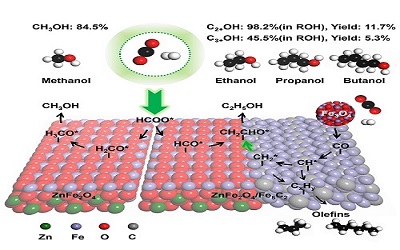
Researchers first synthesized the single-phase crystal structure of ZnFe2O4 spinel. The ZnFe2O4 oxide sample was activated in situ prior to the CO2 hydrogenation reaction.
The experimental observation showed that more than 97% of the reaction products consisted of methanol and ZnFe2O4 catalyst exhibited a high CH3OH selectivity and CO2 conversion. ZnO and Fe2O3 oxides were synthesized and tested for comparison study, which shown a much lower CO2 conversion and CH3OH selectivity.
The results indicated the ZnFe2O4 spinel phase enabled high methanol selectivity during CO2 hydrogenation. The experimental analysis suggested the oxygen vacancy on the surface of ZnFe2O4 spinel is the active site for CO2 and H2 activation.
It was also found that spinel ZnFe2O4 plays a vital role in the formation of oxygenates from CO2 hydrogenation, whereas the formation of the Fe5C2 phase facilitates both carbon chain growth and the insertion of C1 oxygen-containing intermediates to yield C2+OH after further hydrogenation, which greatly increases the selectivity of C2+OH in addition to olefins.
Moreover, introducing Fe5C2 phase to the formation of the ZnFe2O4/Fe5C2 interface greatly promotes C2+OH selectivity in oxygenates to 98.2%, giving a very high C2+OH productivity and an unprecedented C3+OH yield of 5.3%, which surpass the current reported maximum C3+OH yield of 2.1%.
Theoretical calculations further revealed the role of the ZnFe2O4/Fe5C2 interface in the C-C coupling. The ZnFe2O4/Fe5C2 interface drives selective transformation to C2+OH by promoting the facile migration of alkyl species to the interface and coupling with CHO* species, which contribute to the breaking of the Anderson-Schulz-Flory(ASF) distribution and result in the much lower methanol selectivity than expected.
This work demonstrates a new strategy for selectivity tuning in alcohol synthesis by crystal structure engineering.