Research Progress
In a study published in Angewandte Chemie, Prof. WEI Wei and Prof. CHEN Wei from Shanghai Advanced Research Institute of the Chinese Academy of Sciences constructed a hierarchical Cu hollow-fiber penetration electrode (HPE), which helped to realize high-efficiency CO2 electroreduction to C2+ products in both neutral and strongly acidic electrolytes at ampere-level.
The electrochemical conversion of CO2 into carbon-based fuels and valuable feedstocks by renewable electricity is an attractive strategy for both the mitigation of the greenhouse gas effect and the consumption of renewable energy. However, it remains a challenge to possess efficient conversion of CO2 to C2+ products in acidic system due to hydrogen evolution.
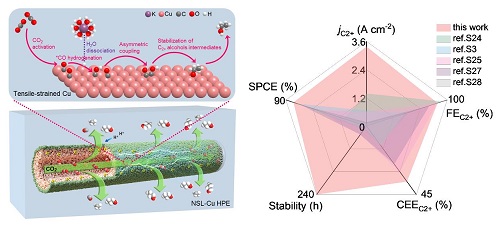
Previous studies by the research team have shown that the Cu hollow fiber permeation electrode can greatly improve the CO2 coverage because of its unique porous structure, thus realizing the generation of C2+ products under ampere-level current under acidic conditions.
To further improve the selectivity of C2+ products at ampere-level current density, researchers constructed in situ lattice strained nanosheets of copper on the surface of Cu permeation electrode by wet chemical/ electrochemical reduction method.
High concentration of K+ could concurrently suppress hydrogen evolution reaction and facilitate C–C coupling. The synergistic effect of lattice strain effect and gas permeability effect enables the Cu permeable electrode to efficiently and stably convert CO2 into C2+ products under both neutral and acidic conditions (Current density > 3.5 A cm-2, C2+ products faraday efficiency >80%).
Experimental measurements, in-situ spectroscopy studies, and density functional theory simulations revealed that tensile-strained Cu HPE boosted the asymmetric C–C coupling to steer the selectivity and activity of C2+ products.