Research Progress
Proton exchange membrane water electrolysis (PEMWE) has emerged as a promising approach in green hydrogen production, owing to its broad power fluctuation adaptability, rapid dynamic response, and high energy efficiency under elevated current densities, which enable effective integration of intermittent renewable energy sources such as wind and solar power.
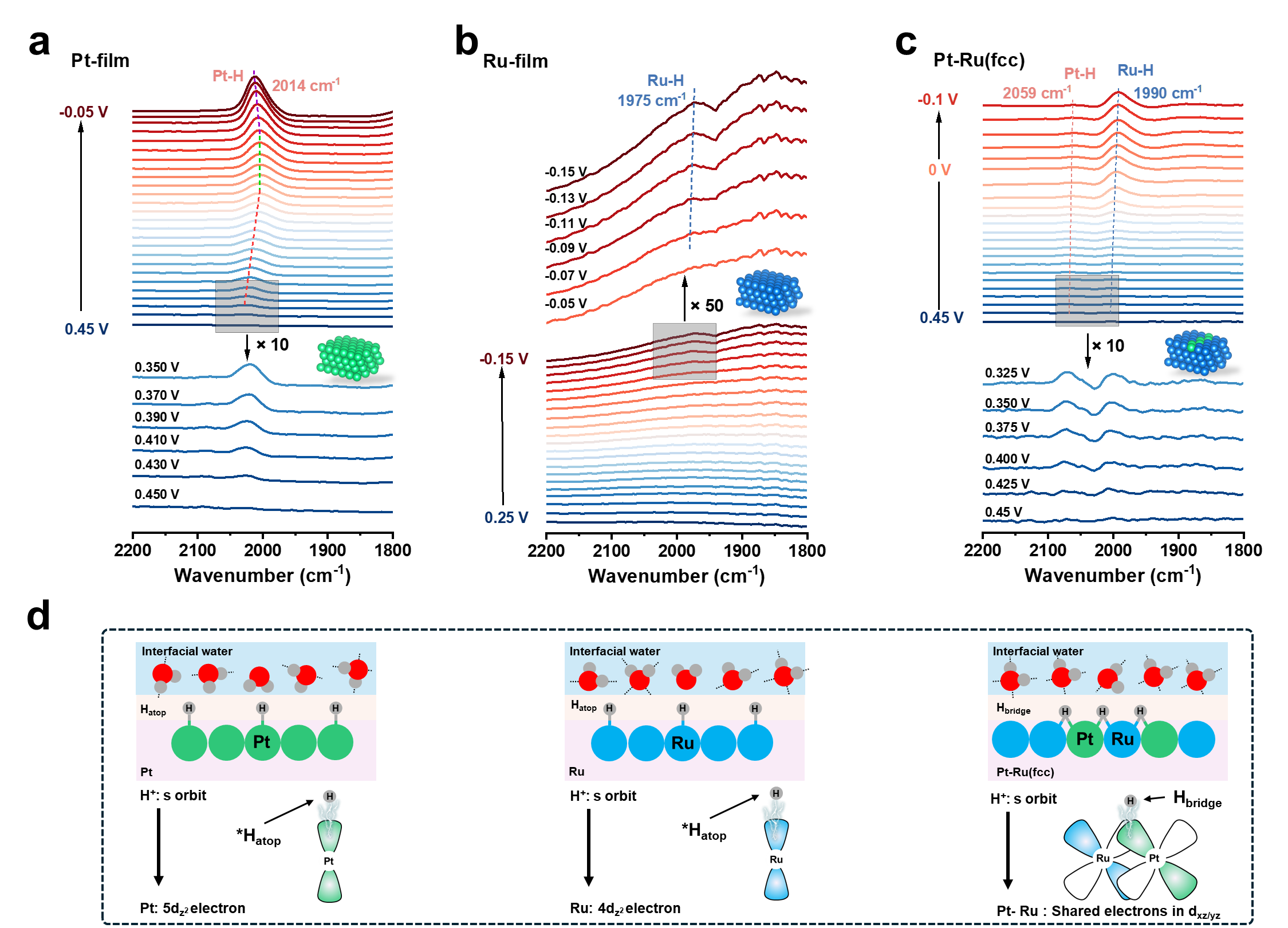
For hydrogen evolution reaction (HER) in acid, atop and multiple adsorbed hydrogen are key intermediates on Pt-group metal. However, the role of bridge hydrogen intermediate (*Hbridge) is oftenneglectedin experiments.
Motivated by this challenge, a research team led by Prof. YANG Hui at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences designed a face-centered cubic-Ru nanocrystal modified with Pt atomic chains and unveiled the important role of *Hbridge intermediates adsorbed on Pt-Ru atomic pairs in determining the intrinsic activity of HER.
The research results were published in Advanced Materials.
The results of in situ attenuated total reflectance surface-enhanced infrared absorption spectroscopy and density functional theory calculations, demonstrate the key role of *Hbridge in enhancing HER catalytic activity and stability. The electrons leap from Pt-Ru pair site to *Hbridge significantly accelerates the Tafel kinetics.
For the HER performances evaluation, the developed Pt-Ru(fcc) achieves an ultralow overpotential of ~ 4 mV at a current density of 10 mA cm−2 under 2.0 μgPt cm-2in three-electrode configuration, and the intrinsic activity at 50 mV is 10.6 times higher than that of commercial Pt/C.
The PEMWE device integrated by advanced fcc electrocatalyst with ultra-low Pt loadings of only 10 μg cm-2, which is 30 ~ 50 folds lower than that used in current industrial PEMWE systems (300~500 μg cm-2), achieves exceptional activity (1.0 A cm-2 at 1.61 V) and remarkable stability (4.0 μV h-1 degradation rate over 1000 h at 1.0 A cm-2). The PEMWE based 80 μm Gore membrane under identical operating conditions requires only 1.54 and 1.58 V to achieve 1.0 and 1.5 A cm-2. Furthermore, the device exhibits negligible decay during the 240 h fluctuating tests.
The identification of the key role of *Hbridge provides mechanistic insights into the activity origin of the Pt–Ru bimetallic catalysts, and opens new avenues for designing next-generation bimetallic catalysts for clean hydrogen energy.
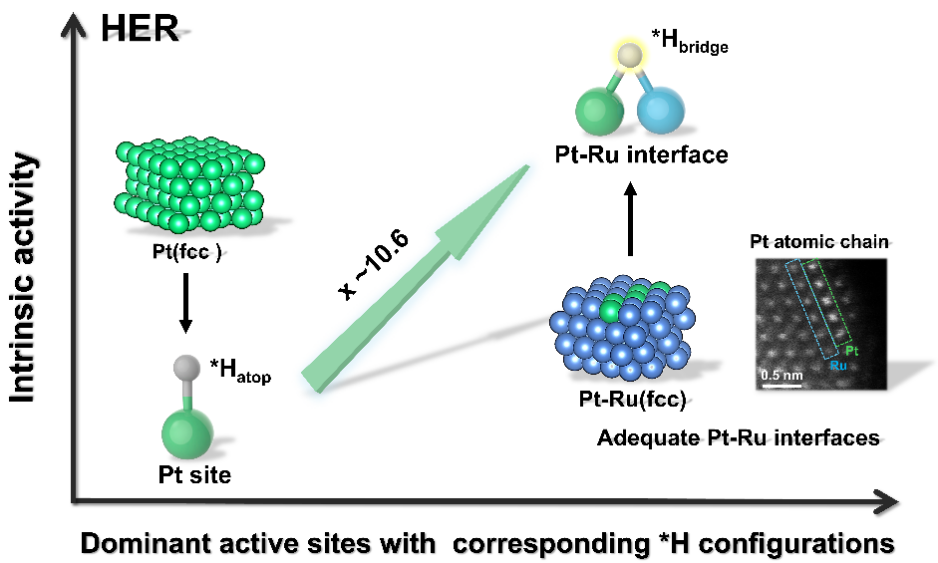
Table of Contents (Image by SARI)
In situ ATR-SEIRAS of hydrogen intermediate (Image by SARI)
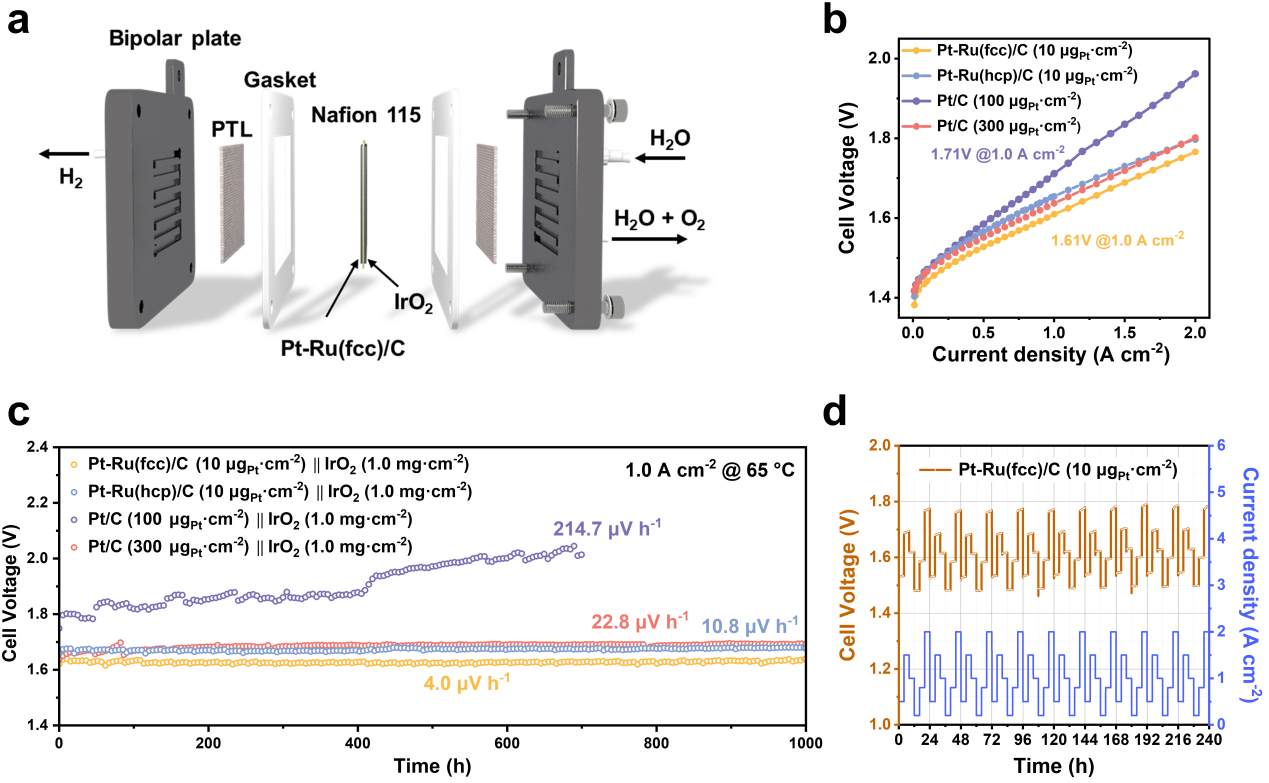
The performance of PEMWE (Image by SARI)