Research Progress
A joint research team led by Prof. ZHANG Lijuan from the Shanghai Advanced Research Institute, Chinese Academy of Sciences and Tongji University proposed a peptide-stabilized lactate oxidase and catalase system, which can effectively deliver oxygen to cells and tissues in Nerve Injury Treatment.
The findings were published in Cell Biomaterials.
Nanobubbles hold tremendous potential in the field of biomedical gas delivery. Their extensive gas-liquid interface and relatively high internal pressure endow them with gas transport efficiency far superior to that of traditional methods.
By investigating the formation, stabilization/diffusion processes and biological effects of enzyme-driven nano-bubbles, the joint research team proposed that the locally high concentrations of gaseous molecules produced in efficient biocatalytic centers and hydrophobic regions within microdomain structures enable enzyme molecules to serve as ideal bioreactors for nanobubble fabrication.
Inspired by enzyme complexes in metabolic processes, researchers constructed a peptide-stabilized lactate oxidase/catalase (LOx/CAT@PNFs) system. This system utilized endogenous substances (lactate and hydrogen peroxide) in living organisms to generate oxygen nanobubbles via enzymatic reactions, and researchers found that the hydrophobic microdomains in peptide nanofibers contribute to the stabilization of these nanobubbles.
“How to detect the formation, chemical identification and evolution of nanobubbles in this system is a big challenge. We detected in-situ the nanobubbles generated by the LOx/CAT@PNFs system and oxygen inside by taking advantage of synchrotron radiation X-ray nanoscale imaging, and then explored their dynamics using the liquid in-situ TEM.” said ZHANG Lijuan, one of corresponding authors.
By regulating lactate metabolism, improving local oxygen supply, and alleviating oxidative stress, the LOx/CAT@PNFs system effectively enhances neuronal survival and improves neural function, demonstrating excellent therapeutic efficacy in both acute and chronic neurotoxic encephalopathy models.
This work not only offers new insights into advancing the application of nanobubble gas delivery systems within biological organisms but also provides valuable research implications for exploring enzyme-driven gas-mediated metabolic regulation mechanisms.
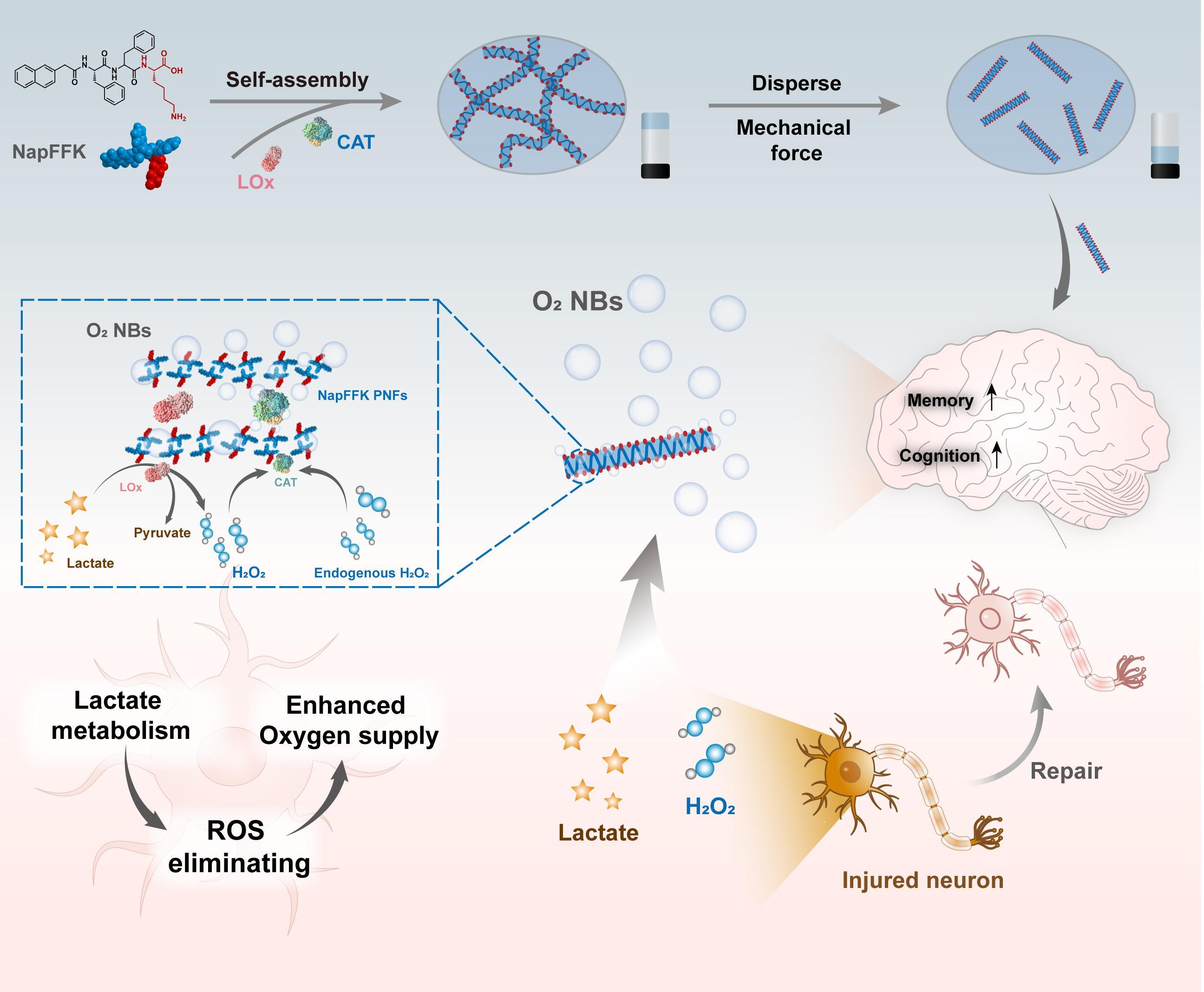
Construction of an efficient biomimetic enzyme complex LOx/CAT@PNFs system and the NB-synergistic metabolic regulation
(Image by SARI)





