Research Progress
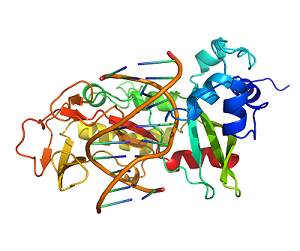
Researchers Reveal Sau3AI Represents a New Subclass of Type IIE Restriction Enzymes by Studying its Crystal Structure
Recently, a research team led by Prof. YU Feng at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences and Prof. HE Jianhua at Wuhan University reported a self-activating mechanism that the Sau3AI C-terminal domain opens the N-terminal catalytic domain through allosteric effects to achieve cleavage of DNA-specific sites.The research results were published in the Structure on August. 30th.
Researchers Develop Target Therapy of Buried Interface for Highly Efficient Perovskite Solar Cells
A research team at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences, collaborated with researchers from the Southern University of Science and Technology and the City University of Hong Kong, reported a facile and effective strategy that is employed for precisely modulating the buried interface through the incorporation of formamidine oxalate (FOA) in a colloidal SnO2-based ETL.The research results were published in the Advanced Materials on July. 17th.
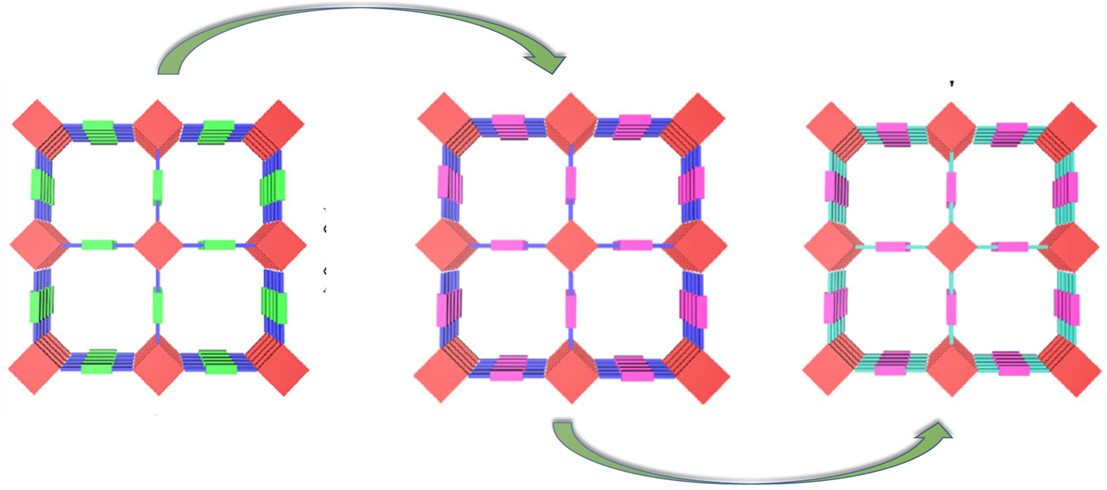
Researchers Construct Post-synthetic Modification of Covalent Organic Frameworks for CO2 Electroreduction
A research team at the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences constructed a multilevel post-synthetic modification strategy to construct catalytic COFs towards CO2RR with high activity and selectivity. The research results were published in Nature Communications.

Scientists Develop a Novel Approach to Support Future Power System
Large-scale clusters of electric vehicles (EVs) are an important reserve measure supporting the construction of new power system. Key technologies towards the reliable and economic operation require collaborative innovation across disciplines and fields.Driven by the increasing needs, a joint research team led by Shanghai Advanced Research Institute of the Chinese Academy of Sciences proposed a novel approach to support future power system towards global net-zero emission target. The results were published in the latest issue of Renewable and Sustainable Energy Reviews.
Novel Approach towards the Fabrication of Artificial GNRs with Pentagon Carbon Embedded
a research team led by Prof. SONG Fei at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a novel approach to precisely fabricate well-defined GNRs with the tailored carbon pentagon embedded inside, supported on the Ag(111) model catalyst. The results were published in Journal of Physical Chemistry Letters entitled "On-Surface Synthesis of Pentagon-Incorporated Graphene-Like Nanoribbons with Multiple Precursors”.
Researchers Develop Highly Efficient Ampere-Level CO2 Reduction to Multicarbon Products via Stepwise Hollow-Fiber Penetration Electrodes
Recent studies have shown that serial hollow-fiber penetration electrodes (HPEs) can improve the CO2ERR performance significantly. To promote the selectivity and current density for C2+ products simultaneously, a research team from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences developed a stepwise CO2ERR strategy using Ag and Cu HPEs to reach high-rate C2+ production.The results were published in Appl. Catal. B-Environ. on 28th May, 2023.





