Research Progress
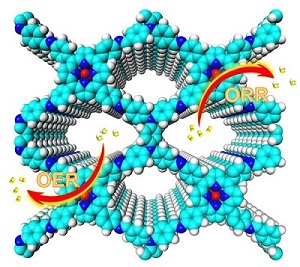
Researchers Constructed Bifunctional Catalysts with Redox-active Sites for Oxygen Reduction and Oxygen Evolution Reaction
Recently, a research team led by Prof. ZENG Gaofeng and Associate Prof. XU Qing at the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences constructed a novel bifunctional COFs towards ORR and OER by combining redox-active units with catalytic centers in the frameworks.
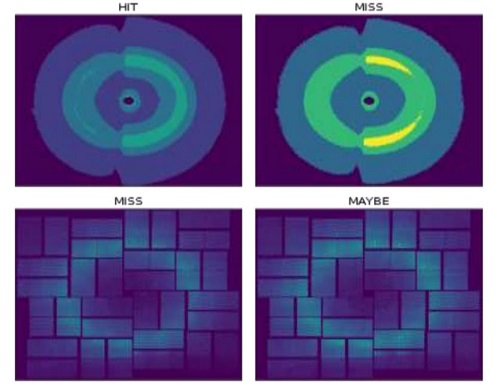
Researchers Propose a Novel Federated Learning-Based μXRD Image Screening Method
A research group at the Shanghai Advanced Research Institute, Chinese Academy of Sciences unveiled the solutions to improve the screening while protecting data privacy.The results of the physics law-informed federated learning (FL) based μXRD image screening method are published on IEEE TRANSACTIONS ON INDUSTRIAL INFORMATICS.
Researchers Propose a Novel FTO Route with Ultrahigh Carbon Efficiency
A research team led by Prof. ZHONG Liangshu at Shanghai Advanced Research Institute (SARI) of Chinese Academy of Sciences reported a non-classical Fischer-Tropsch to olefins (FTO) process featuring high carbon efficiency that realizes 80.1% olefins selectivity with ultralow total selectivity of CH4 and CO2 (<5%) at CO conversion of 45.8%.
Researchers Develop Synergistic Catalysts of Ru single-atoms and Zeolite for High-efficiency Hydrogen Storage
A research team led by Prof. CHEN Xinqing at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences developed a new strategy utilizing Ru single atoms and *BEA zeolite to synergistically catalyze hydrogen storage of liquid organic hydrogen carriers (LOHCs) with superior performance.
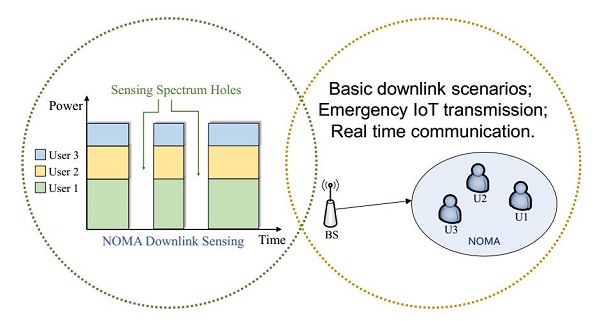
Researchers Develop a Novel Spectrum Sensing Technique for 6G-Oriented Intelligent IoT Communications
A joint research team creatively proposed a novel spectrum sensing technique for 6g-oriented intelligent IoT communications, seeking a feasible way to provide the underlying support for perceptual interference and intelligent identification between large-scale coexistence and aliasing IoT users in future 6G scenario.
Researchers Proposed a Facile Tactic to Enhance Electrocatalytic Conversion of Carbon Dioxide
A research team from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a facile tactic that enables exceedingly efficient CO2 electroreduction to CO by virtue of low-coordination chloride ion (Cl-) adsorption on a silver hollow fiber (Ag HF) electrode.





