Research Progress
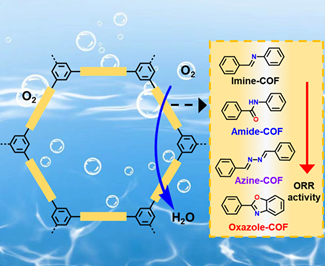
Researchers Developed Linkage Engineering of Covalent Organic Frameworks for the Oxygen Reduction Reaction
A joint team lead by Prof. ZENG Gaofeng and Associate Prof. XU Qing from Shanghai Advanced Research Institute, CAS and Prof. JIANG Zheng from University of Science and Technology of China employed catalytic linkage engineering to modulate the catalytic behaviors and found the catalytic performance is determined solely by the electron states of carbon atoms in the linkages. The research results were published in Angew Chem Int Ed.
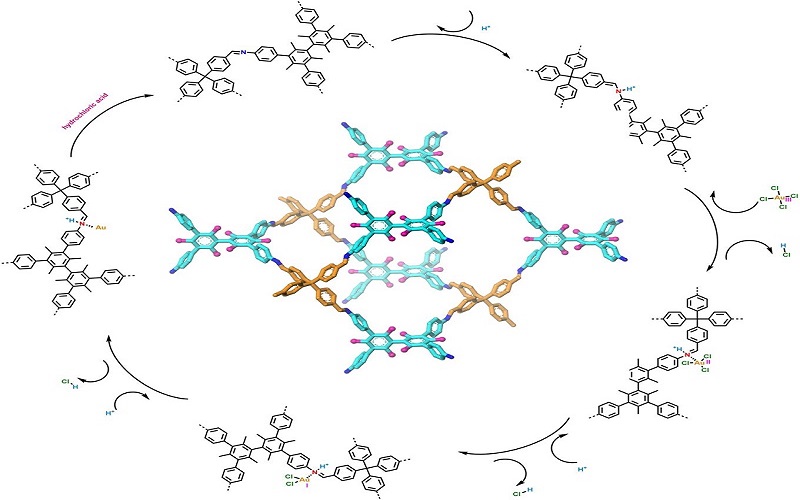
Researchers Constructed Noninterpenetrated Three-dimensional Covalent Organic Framework for Au Ions Capture
A research team led by Prof. ZENG Gaofeng and Associate Prof. XU Qing at the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences constructed a novel non-interpenetration 3D COF towards Au ions capture by imine bonds in the frameworks. The research results were published in Adv. Funct. Mater. on Apr. 23rd.
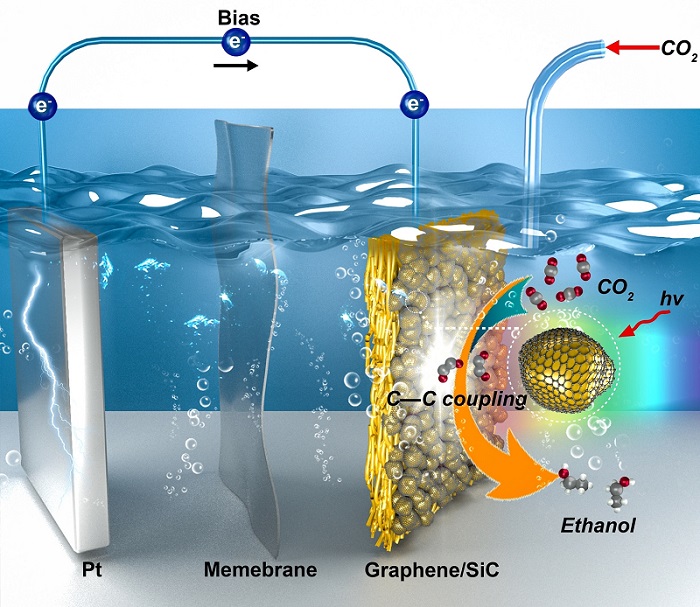
Researchers Developed a Novel Graphene/Silicon Catalyst for Highly Selective Photoelectroreduction of Carbon Dioxide to Ethanol
a research team led by Profs. CHEN Wei and WEI Wei from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported efficient CO2 photoelectroreduction to ethanol (C2H5OH) over graphene/silicon carbide (SiC) catalysts under simulated solar irradiation with ethanol (C2H5OH) selectivity of >99% and a CO2 conversion rate of up to 17.1mmol gcat-1h-1. The results were published in Angew. Chem. Int. Ed.
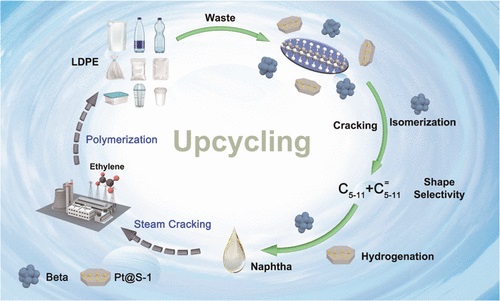
Researchers Propose an Effective and Reusable Tandem Catalyst for Plastic Waste Conversion
a research group led by Prof. WANG Hui and Associate Prof. LUO Hu at the Shanghai Advanced Research Institute, Chinese Academy of Sciences developed a tandem catalytic reaction to efficiently convert low-density polyethylene (LDPE) into naphtha, where a naphtha yield of 89.5% is obtained with 96.8% selectivity of C5?C9 hydrocarbons at 250 °C, catalyzed by mechanically mixed β zeolite and Pt@S-1 catalysts.The research results were published in Journal of the American Chemical Society recently.
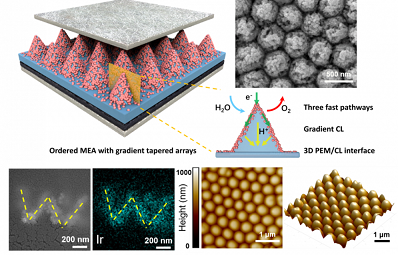
Researchers Propose a Novel Ordered MEA for High-performance PEMWE
a research team led by Prof. YANG Hui at Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences reported a novel ordered MEA based on anode with 3D membrane/catalytic layer (CL) interface and gradient tapered arrays, which not only greatly increases the electrochemical active area but also decreases the overpotentials of both mass transport and ohmic polarization.The research results were published in Nano Letters recently.

Researchers Design a Novel Copper Gas Penetration Electrode to Efficiently Reduce CO2 to Multicarbon Products
a research team from the Shanghai Advanced Research Institute of the Chinese Academy of Sciences reported a hierarchical micro/nanostructured Cu(100)-rich hollow-fiber gas penetration electrode (GPE), breaking through the bottleneck of low CO2 solubility limit and realizing electrochemical reduction of CO2 to multicarbon products under ampere level current density.





